Topic in Focus
Scientific findings from and uses of embryo research
Image: PINO NOA – Pia Bublies & Nora Coenenberg, Hamburg
Early human embryos are microscopic cell clusters that can also be created in a laboratory, during artificial insemination for example. Research on embryos can provide important scientific findings into how human life develops in the initial days following fertilisation. These findings can be useful for the identification and treatment of diseases and help make reproductive medicine safer.
Embryo research uses embryos that are only a few days old and have been created during reproductive treatment and not yet transferred to the uterus. These embryos are often referred to as pre-embryos – microscopic cell clusters resulting from the fertilisation of a human oocyte. Creating these early embryos in vitro, i.e. in the laboratory, for purposes such as artificial insemination has been possible for over 30 years. Embryo research is a recognised field and is practised internationally.
When a sperm fertilises an oocyte in natural conditions (in vivo), the resulting embryo can implant into the uterus six to ten days following fertilisation. During this phase the body carries out a multi-stage quality inspection: It becomes clear whether the combination of the mother’s and father’s genetic material is viable and whether the embryo is able to develop into a human being. approximately every second embryo dies in the first few days following fertilisation because chromosome missegregation or mutations have made it inviable. It is estimated that, in natural conditions, only every third or fourth fertilised oocyte leads to a live birth. In the case of in vitro fertilisation, approximately only every fourth embryo is viable at present.
Research on early human embryonic development delivers important scientific findings on human developmental biology. The hope is to find new ways to improve the treatment of infertility, miscarriages, premature births, and genetic diseases or deformities in children, or prevent them entirely.
“Some research questions can only be answered with the help of human embryos.” (German only)
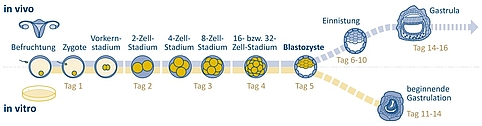
Early embryonic development
During fertilisation a sperm cell penetrates the oocyte in the woman’s fallopian tube. Both the sperm cell and the oocyte contain a set of chromosomes. In vitro, i.e. in artificial conditions in a laboratory, the sperm cells can either penetrate the oocyte independently or be injected. When the oocyte and sperm cell fuse, a cell with a full (double) set of chromosomes is formed: the zygote. The parental genomes initially remain in their respective pro-nuclei, which are formed from the nucleus of the sperm cell and oocyte.
Figure 1: Process of early human embryonic development inside the woman’s body and in vitro under laboratory conditions. In vitro research on “surplus embryos” is permitted up until day 14 under strict conditions in some countries, such as the USA, Israel, the United Kingdom, France, and Japan. This type of research is currently prohibited in Germany. | Design: Emde Grafik
Now cell division begins. Observations of mouse embryos suggest that a complete mixing of the parental chromosomes first takes place in the 2-cell stage. The membranes of the pre-nuclei have to dissolve for this to occur. The resulting nuclei contain both the maternal and paternal sets of chromosomes.
In the 4-cell stage the embryonic genome is activated. This is essential for protein synthesis and continuing cell division. After a further cell division into the 8-cell stage, the cells begin to lose their totipotency and become pluripotent during the cellular differentiation which now begins. Pluripotent cells have the potential to differentiate into any of the tissue types found in the body. Unlike totipotent cells, they are no longer capable of developing into a complete organism.
If the cell sphere consists of 16 or 32 cells it is referred to as a morula. This sphere divides further and reaches the uterus around four days after fertilisation in natural conditions. Some of the cells move towards the centre of the morula, while the rest remain on the outside. On day five the embryo consists of around 60 to 100 cells. The cells become flatter and more compact, eventually forming a hollow sphere with two cell layers: the blastocyst.
During in vitro fertilisation (IVF) this is usually the stage at which the embryo is transferred into the uterus. The fact that the human embryo initially finds itself outside of the human body for up to six days and is thus exposed to artificial growing conditions is unique to IVF.
In order to continue growing, the blastocyst must implant itself into the uterine wall between days six and ten. The embryo can only develop into a foetus in the uterus. The embryo becomes known as a foetus from day 70, when all organs and limbs have formed. First, gastrulation of the embryo begins after a few days: The germ layers form. The new human organism (also known as “embryo proper”) develops from these germ layers and the surrounding cells form the extra-embryonic membranes and placenta. It is now possible to induce gastrulation in artificial conditions. During in vitro gastrulation, the embryo reaches around day eleven of natural development and is then 0.3 millimetres in size.
“Research objectives of outstanding interest also include fundamental research.” (German only)
Development of reproductive medicine
In addition to resolving fundamental matters concerning embryonic development and the early stages of diseases, embryo research can also help provide answers to important questions in reproductive medicine. It can be used to find better fertility treatments, to improve the survivability and healthy development of embryos and foetuses during pregnancy, and to help prevent miscarriages and premature births.
An example in this case is the research on abnormal numbers of chromosomes (aneuploidy) in early embryos in some or all of their cells. Aneuploidy is found to be the cause of around one in three investigated miscarriages, making it the most common cause of spontaneous pregnancy loss. Scientists require a better understanding of the causes behind this high rate of aneuploidy in human embryos in order to provide advice and treatment to couples experiencing infertility or who have suffered repeated miscarriages.
Based on results from international research, it has already been possible to improve reproductive medicine and make it safer. An example of this is elective single embryo transfer (eSET) in the case of artificial insemination. During this procedure, a larger number of oocytes are fertilised and the embryo with the greatest chance of development is transferred into the uterus. This results in a major reduction of risky pregnancies while keeping the chances of success roughly the same. In the United Kingdom, these research findings have systematically been used to improve the quality of IVF treatment. As a result, the number of multiple pregnancies and the associated serious risks of premature labour for both mother and child have been significantly reduced over a span of ten years. The twin rate per IVF in the United Kingdom is approximately 10 percent thanks to the eSET procedure. In Sweden it is only 4 percent. In Germany, where the procedure is prohibited, approximately every fifth IVF treatment results in a twin pregnancy.
Stem cells in personalised and regenerative medicine
Due to their pluripotency, human stem cells from early embryos (hES cells) can develop into every type of cell and form all kinds of organism tissue types. This means they have a great potential for regenerative and personalised medicine. The first stem cell lines were successfully generated from human embryonic stem cells in the late 1990s. Since then, there has been great hope for research using these cell lines, particularly in the areas of cell replacement therapy, i.e. the repair of damaged tissue. Clinical studies on the treatment of age-related macular degeneration, a form of severe visual impairment, are currently being carried out using cell therapy, for example. Research is also looking to hES cells for the treatment of common illnesses such as diabetes, arthritis, cardiac arrest and strokes. The in vitro embryo does not survive the generation of hES cells, and the procedure is currently prohibited in Germany. However, it is possible to import embryonic stem cells from other countries under tight legal restrictions.
Existing hES cell lines are sometimes cultivated over years and have accumulated genetic and epigenetic alterations in the process. They may also be contaminated with pathogens such as prions or mycoplasma. It would therefore be necessary to generate new hES cell lines under clearly defined conditions for use in clinical applications.
Fundamental research on gene therapy
Another crucial field of medical research is the use of genome editing to genetically “correct” hereditary diseases. There are two different approaches, depending on the disease. The first involves genetically correcting body cells (somatic cells) and the second involves editing the embryonic genome in vitro. Somatic gene therapy has already been part of clinical tests for years. Usually it is only used for the treatment of an individual person, who in most cases is already unwell.
Modifications to the germline take place when oocytes and sperm cells or their precursors are exposed to gene therapy. These modifications could have potential effects on future generations as the genetic modification can be inherited. The overwhelming majority of the international scientific community agrees that it is currently not justifiable to use germline interventions to pursue the objective of bringing a person into the world, due to the as yet unknown risks and given the availability of possible alternatives such as somatic therapy, pre-implantation diagnostics, and adoption. In 2018, twins were born in China that had had their genes specifically modified by means of the CRISPR-Cas9 technique during fertilisation. This case met with outrage from the scientific community.
Fundamental research on germ and somatic cells and on early human embryos would be useful, at the very least for the purpose of critically examining and evaluating the opportunities and risks of this type of gene therapy. Such research could also improve the assessment of and possibly even reduce the off-target effects still associated with this method, such as extensive DNA-sequence loss and chromosome loss.