Topic in Focus
Vaccine Development, Testing, and Approval
Illustration: Emde Grafik
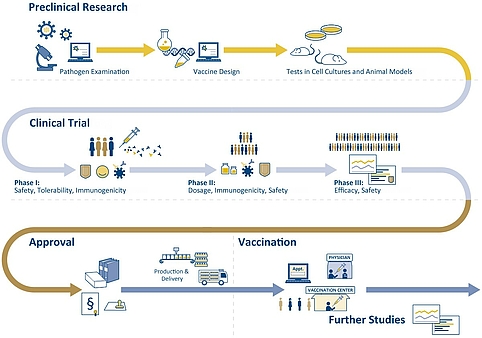
The development of a vaccine may take up to 15 years and cost several hundred million Euros. It all begins with basic research, in which scientists first identify which of the body’s defenses a pathogen triggers. They then investigate how this defense can be activated without infection through the pathogen. Once the mechanisms are known, a vaccine must first pass laboratory and animal experiments before it is clinically tested on the human organism.
Efficacy and tolerability of a potential vaccine are first tested preclinically in laboratory experiments and in animal models. Animal experiments are mandatory for the development and approval of drugs at the national or European level – primarily to identify side effects. All necessary data for an approval are specified. Vaccines against COVID-19 are first tested, for example, on mice and rats. In addition to side effects, also immune response, dosage, and vaccination regimen are investigated. Through these experiments, initial knowledge is gathered, and the risks for test subjects in clinical trials are minimized.
Figure 2: Schematic representation of vaccine development and delivery. Sources: vfa, NaLI | Illustration: Emde Grafik
Phases of clinical trials
The clinical trials are divided into three phases. In phase I, a new vaccine is tested in less than one hundred healthy adults. Here, the main focus is on safety and tolerability, i.e., whether the vaccine is free of severe adverse effects. Phase II trials usually involve several hundred volunteers. This is where the immune response and the protective effects are investigated, and the dosage is optimized. Phase III involves several thousand individuals. In this phase, significant proof of efficacy of the new vaccine must be demonstrated. Vaccination schedules will be reviewed, and potential differences in efficacy that may occur depending on age groups or gender will be investigated. A new vaccine is consistently tested for safety in all three phases.
Only after completion of all three clinical phases without complications can the vaccine be submitted for approval. All vaccines or drugs to be administered in Germany require national approval or approval from the European Commission based on the recommendations of the European Medicines Agency (EMA). The prerequisite is a favorable benefit-risk ratio: the benefit of vaccination must be greater for the general public than the risk due to possible side effects or long-term effects on the individual.
“For many of these new vaccines, the first priority is to demonstrate safety and tolerability. You have to remember that a vaccine is used to treat healthy people, so it has to be very safe first and foremost. And then you have to show that it also protects. That is, from infection and from the disease it triggers. Third, and this is the crucial point, you have to know how long such protection will last. Of course, this can only be observed over a long period of time, a year or more. That means you have to vaccinate and then wait and see how immunity develops in real life, so to speak.”
Further testing after approval
In Germany, the Paul-Ehrlich-Institut (PEI) is responsible for the national approval and monitoring of vaccines. At the Federal Institute for Vaccines and Biomedicines, vaccines are continuously monitored for safety, efficacy, and tolerability after approval. This involves checking the adverse effects, which are reported to the PEI by the manufacturers and physicians. Patients can also report side effects themselves online. In such cases, the PEI investigates whether and to what extent there is a connection with the administered vaccine. In addition, long-term effects are evaluated since no reliable statements can yet be made within the approval trials framework. The PEI also forwards the reports to the European Medicines Agency (EMA), which compiles and compares the EU member states’ data.
“After phases I, II, and III of clinical trials, there is actually also a phase IV trial. Here the vaccine is already being broadly used. However, there is still a focus on gaining as much information as possible. To check if there are any side effects at a later stage, for example after 10 months, and also to see how long the protection lasts. Now, these are not strictly controlled studies. We are talking about everyday people who have been vaccinated. But we will nevertheless find out if these people report this or that problem. Or we might learn after, say, 12 months that a person was definitely vaccinated but still gets sick or is infected. That is why it is so important to determine long-term effects. That is why we have to continue checking after the approval.”
Funding and equitable distribution
Basic research for vaccines in Germany takes place at universities, at state authorities such as the Paul-Ehrlich-Institut (PEI) or the Friedrich-Loeffler-Institut (FLI), and at institutes of the Helmholtz Association, the Leibniz Association, the Fraunhofer-Gesellschaft, or the Max Planck Society. Their research is financed by public funds. In addition, foundations such as the Bill and Melinda Gates Foundation or the Wellcome Trust are involved.



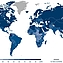
Figure 3: National measles vaccination coverage rates in 2019, i.e., administration of both doses of measles vaccine MCV2 needed for full protection by the nationally recommended age. By the end of 2019, 71 percent of children worldwide received two doses of measles vaccine according to national immunization schedules. Source: WHO | Illustration: Emde Grafik
When it comes to vaccines, there is always critical discussion about equitable distribution and fair prices. The Global Alliance for Vaccines and Immunization (GAVI) was founded in 2000. It is one of the world’s largest customers of vaccines and commits itself to an easier access to vaccines for developing and emerging countries. Governments of many countries are represented in the alliance, including the German government, the WHO, the United Nations Children’s Fund UNICEF, the World Bank, the Bill and Melinda Gates Foundation, vaccine manufacturers, and non-governmental organizations.
“One possibility has been introduced by forming the “Global Alliance for Vaccines and Immunization”, or Gavi for short. This organization is backed by many countries, including Germany, and by foundations such as the Gates Foundation. They basically distribute the vaccines to the poorest countries free of charge or at a low cost and cover the expenses. Of course, it must also be ensured that the price level is appropriate, which can certainly be achieved through competitions, and that is exactly how it’s done. It is also possible, and this was done to some extent with the COVID-19 vaccines, to already say today: We will support you, and we will also buy 300 or 500 million dosages later if the vaccine works. In other words, we are already guaranteeing the purchase of the prospective vaccine. This is called Advanced Market Commitment. It gives research and development an incentive and pushes the price down.”
Recommendations instead of mandatory vaccination
At the global level, the WHO makes recommendations on which vaccinations are appropriate for specific population groups. For the European Union (EU), there is the European Vaccination Action Plan (EVAP). In Germany, vaccination recommendations are published by the Standing Committee on Vaccination (STIKO). This committee is staffed with independent scientific experts who are appointed by the Federal Ministry of Health and the state health authorities. The STIKO recommendations form the basis for the National Immunization Plan (NIP). In this plan, the federal and state governments define targets for vaccination rates and specify implementation strategies and responsibilities.
Mandatory vaccination does not exist in Germany because vaccination affects the right of physical integrity, which is particularly protected under article 2 of the Basic Law of the Federal Republic of Germany.
“We have said that it would simply not be possible to justify mandatory vaccination for everyone because that would pose an interference with self-determination in regard to one’s own body, which is not justifiable. It depends on different things, for instance on the respective distribution of risk in the overall population. That is a relevant issue, especially in the case of COVID 19. That’s why we also said that in this case, a general obligation to vaccinate – not to mention the question of enforcement, so to speak, which is something that is also discussed: what would something like that even look like – we relatively quickly and clearly ruled it out due to ethical reasons.”
In Germany, legislation can stipulate that certain groups of people have to provide proof of immunity or vaccination against highly contagious diseases – as is the case with measles. According to the Measles Protection Act of 2020, children must prove vaccination or immunity against measles before being admitted to a daycare center or a school. This also applies to employees in medical practices, hospitals and daycare centers, teachers, educators, as well as residents and employees in refugee shelters.
The German Ethics Council also recommended this approach in 2019. In its statement, “Vaccination as an obligation?”, it spoke of a general moral obligation to vaccinate against measles but recommended the introduction of a statutory obligation to vaccinate only for occupational groups with special responsibilities.
“We have actually formulated very clearly that there is a moral obligation for everyone to vaccinate against measles. For certain occupational groups, we have also formulated that there are reasons for mandatory vaccination. Many people don’t know that measles are extremely contagious, much more contagious than COVID-19. It is a highly contagious disease that can be quite severe and even fatal in certain groups. Especially in certain patient groups, a measles infection can have very, very severe long-term consequences, including anencephaly, in which the brain is virtually destroyed altogether. So we said, this is a constellation where it is justified to think about such an occupation-specific vaccination obligation for certain occupational groups who work with vulnerable people, and who could actually endanger them.”
Vaccine confidence in the population
The success of vaccination depends on the public’s confidence in its efficacy and safety. Therefore, it depends on transparent communication of the effects of vaccines. Globally, this is an enormous challenge. WHO has in 2019 identified vaccine hesitancy as one of the ten major threats to global health.
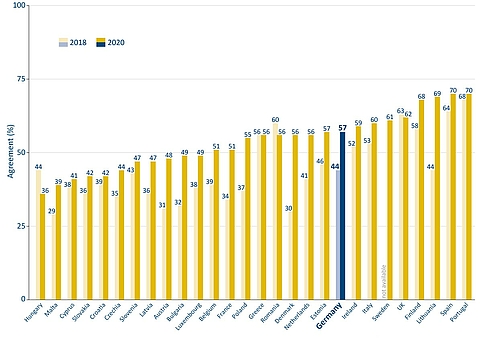
In Germany, general vaccination opponents are a minority. A large proportion of the population considers vaccinations to be very important in principle. According to a representative survey conducted by the Federal Centre for Health Education in 2018, 77 percent of people between the ages of 16 and 85 can be described as vaccination supporters, 17 percent have partial reservations, and 6 percent reject vaccination. The proportion of vaccination supporters has thus increased in recent years. In 2012, it was still at 61 percent. In most other European countries, the willingness to be vaccinated has also increased in recent years, according to surveys conducted on behalf of the European Commission.
Figure 5: General vaccine confidence of the population in EU countries and the UK according to 2018 and 2020 surveys. Source: Vaccine Confidence Project, European Commission | Illustration: Emde Grafik
In Germany, there is a particularly high level of trust in established vaccinations against childhood diseases. The majority of parents consider these vaccinations for children to be important, and vaccination rates are correspondingly high. By the time children enter school, more than 90 percent have received the vaccinations recommended by the STIKO. Vaccinations against childhood diseases have been established for decades, the vaccines have been in use for a long time, and knowledge about the benefits of vaccinations is widespread. The vaccines are trusted, and their safety is proven.
In the case of vaccinations against seasonal influenza, the WHO’s and the European Commission’s target of a vaccination rate of 75 percent in the age group 60 and older has not yet been reached in Germany either. According to the STIKO, only around 35 percent of this group was vaccinated in the 2018/2019 season. During the COVID-19 pandemic, the willingness to be vaccinated against influenza increased. People want to avoid getting sick in light of the strained healthcare system.