Topic in Focus
Standards and practices of embryo research
Image: Adobe Stock / sinitar
There are no binding international conventions regarding research on human embryos. It is permitted to a limited extent in numerous countries. Usually, research may only take place up to 14 days following fertilisation on embryos that have been fertilised outside of the human body. Researchers have now managed to generate embryo-like cell formations from stem cells.
International and national regulations
Neither the United Nations nor Europe as a whole have established uniform rules or regulations for conducting research on human embryos or on human embryonic stem cells (hES cells) derived from human embryos. However, various statements and regulatory efforts have been made which are relevant to the feasibility and possible objectives of research on human embryos.
“The Act for Protection of Embryos equates embryos which are a few days old and a born human in terms of human rights and protection of life.” (German only)
Passed in 1990, the Act for Protection of Embryos (Embryonenschutzgesetz, ESchG) prohibits research on early human embryos outside of the human body in Germany. This means that scientists working in Germany can only make limited contributions to answering important research questions concerning human embryonic developmental biology. Generating stem cells from human embryos (hES cells) is prohibited under the same law. On the other hand, the German Stem Cell Act (Stammzellgesetz, StZG) permits, under certain conditions, the import of embryonic stem cells generated abroad for research objectives of outstanding interest.
“In Germany we benefit from embryonic stem cells generated abroad.” (German only)
In vitro research on early human embryos is permitted under strict conditions in countries like the USA, Israel, Sweden, the United Kingdom, France, and Japan. In those countries, research may only be conducted on “surplus embryos” for 14 days following fertilisation. After this, the embryos must be discarded. “Surplus embryos” refers to embryos that were originally created for artificial insemination and then are no longer used for this purpose. Some of these countries permit embryos to be generated from gametes donated specifically for research purposes. As a general rule, justification is required as to why the available surplus embryos are insufficient in number or unsuitable for the research project in question.
The 14-day limit as a cut-off point for in vitro research on embryos is based on the bioethical recommendations made in the 1984 Warnock Report, prepared under the direction of British philosopher Mary Warnock. The proposal was widely accepted at the time, due to the fact that even naturally fertilised early embryos often die before implantation and that implantation marks the start of pregnancy. In addition, in vitro embryos at the time were not able to survive longer than five to six days when cultivated in the laboratory. As it is becoming increasingly possible to cultivate in vitro embryos beyond 14 days, an extension of this cut-off limit to 28 days is being discussed internationally in order to enable scientists to conduct more research into and develop treatments for the causes of issues such as miscarriage and of diseases such as congenital heart defects and disorders of the central nervous system. Supporters of this extension argue that embryos do not have any kind of sensitivity as by this stage they still have not developed any functional nerve connections or sensory systems.
Some research questions can only be addressed using embryos produced from sperm cells and oocytes donated for research purposes. The experimental approach in this case directly concerns the fertilisation process itself. Donated surplus embryos, on the other hand, have already completed certain processes, such as fertilisation itself, the formation and migration of the pre-nuclei, and the replication of the parental genomes in the pre-nuclei.
Alternative stem cell research method
To date, many scientific findings on embryonic development have only been derived from animal experiments, mostly on mice. However, reliable results regarding the development of human life, the epigenetic, genetic, and environmental causes of diseases, and the specific development of appropriate treatments cannot be gained exclusively from animal experiments. It has been found that many specific biological processes differ between humans and animals, for example the fertilisation of the oocyte, cell division at the time of activation, and the regulation of certain genes.
Induced pluripotent stem cells (iPS cells) present a potential alternative to the generation of human embryonic stem cells (hES cells). Around 15 years ago, researchers first succeeded in turning fully differentiated adult somatic cells into induced pluripotent stem cells. iPS cells can be produced by reprogramming adult skin or blood cells from donors or patients. However, there is some evidence to suggest that iPS cells exhibit certain genetic and epigenetic differences to hES cells: iPS cells can retain epigenetic patterns from the adult cells from which they are generated. These differences and the question as to what extent the differentiation of hES and iPS cells in vitro correspond to embryonic development in vivo are an object of embryo research. On the other hand, mutations which accumulate in the original adult cells in the course of a lifetime are passed on to the iPS cells. A better understanding of cellular differentiation processes necessitates a comparison of the iPS cells with the corresponding stem cells in the embryo’s cell cluster and with hES cells. Such studies could also provide insights into the potential of iPS and hES cells for research into diseases and the development of cell therapies.
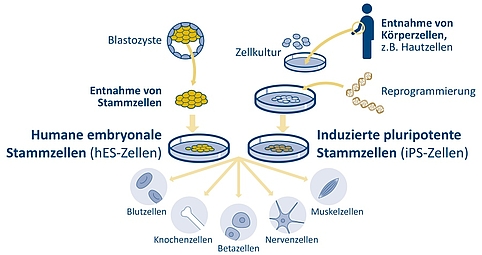
Figure 2: The inner cell mass of the blastocyst contains human embryonic stem cells (hES). These can be removed and cultivated in vitro. They are pluripotent. This means that, depending on the cultivation conditions, they are able to differentiate into organ-specific cell types, or into different types of blood, bone, nerve or muscle cells, or insulin-producing beta cells. Some years ago researchers discovered how, by means of genetic reprogramming, induced pluripotent stem cells (iPS cells) could be produced from adult human skin or blood cells. | Design: Emde Grafik
In recent years, scientific progress has resulted in the lines between somatic cells, gametes and embryos becoming increasingly blurred. International stem cell research has since created various cell formations (embryoids) with embryo-like features. This is how researchers succeeded in producing different embryo-like structures, such as blastocyst-like models (blastoids). In 2023, for the first time, scientists successfully produced advanced stages of development from human embryonic stem cells (hES cells) without a fertilisation process having to take place. These stages usually correspond to those that develop shortly after the embryo’s implantation into the uterus. The developmental potential of these cell formations is yet to be determined – in particular because their development in vitro independently comes to an end after a few days, and it would not be ethical to implant them into a human organism. Comparative research on embryos could also provide insights about this potential.
Published: May 2021, updated November 2023
Previous Page: Scientific findings from and uses of embryo research