Topic in Focus
Modifying Plants Genetically – An Overview of Methods

Image: Adobe Stock / vitstudio
Humankind has always been inventive in its efforts to achieve better agricultural yields. It has already influenced the genetic material of cereals and other crops by selecting beneficial naturally occurring genetic modifications. Genetic engineering makes it possible to transfer hereditary characteristics in a targeted manner. Genome editing specifies and simplifies such applications.
Humans have been systematically practicing agriculture for around 12,000 years. The first plant breeders lived in Mesopotamia in the Near East between the Euphrates and Tigris rivers. Today, most of this area belongs to Iraq and Syria. As far as we know today, humans settled permanently in this region for the first time and cultivated, for example, barley, emmer and ancient types of wheat. By using individual plants – for example, those whose seeds tend to set late – for the next sowing, selection took place through human hands.
By doing so, genetic changes were used and advanced long before the first knowledge about genetic material or molecular biological processes in cells emerged. For mutations occur time and again even without human intervention. They occur within all living organisms due to replication errors, transmission and repair of the genome, or as a result of environmental influences such as solar radiation and oxygen radicals on normal metabolism. While most of these gene changes have no significant effects, some are even detrimental to the survival of the plant and the transmission of genetic information to the next generation. Only less than one per cent of natural mutations result in beneficial new traits. This is one of the driving forces of evolution.
Stone Age farmers also discovered the benefits of crossbreeding cereals with related species, such as wild grasses. Today’s molecular biology studies show that people have been changing the genetic material of cultivated plants for thousands of years through traditional breeding. Not only did mutations occur at individual points, but entire parts of the genome were lost or placed elsewhere. It has also been proven that some gene sequences multiplied during the course of breeding – and that gene segments from unrelated species were inserted.
“Plants are stationary creatures and must constantly adapt to growth conditions. It is through mutations that they succeed, which makes plants very flexible, and people have been taking advantage of this since they settled down thousands of years ago. For example, they have domesticated wild species, and they have also improved plants through breeding. Our current cultivated species have been continuously adapted to the desires of humans. Today’s plants are therefore adapted to the framework conditions of agricultural production.”
Advances in plant breeding
Plant breeding started to become more systematic at the end of the 19th century with the findings on crossbreeding genetics by Gregor Johann Mendel (1822–1884). The Austrian Augustinian priest and naturalist had clarified the laws of heredity after years of experimentation with pea plants in the garden of St. Thomas Abbey in Brno in today’s Czech Republic. Mendel recognised that there are two copies of each hereditary trait in peas – i.e. one from each parent plant. And he observed that dominant and recessive traits exist – i.e. those that always prevail and others that do not always manifest themselves. Although he is now regarded as the “father of genetics,” his groundbreaking findings were hardly recognised during his lifetime. It was not until the beginning of the 20th century that his laws of heredity were rediscovered and their implications recognised.
With the knowledge of the rules of inheritance, plants could be bred in a more targeted way. The establishment of cell culture techniques at the beginning of the 20th century, which enabled selection in the laboratory, and hybrid breeding were further advances. In hybrid breeding, two purebred parent lines are crossed with each other – i.e. those in which both copies of a gene are identical. In certain cases, this produces a particularly robust and high-yielding new generation. Their offspring, however, no longer show the positive traits in all individuals. Therefore, farmers have to produce or buy new hybrid variety seeds every year.
Mutation breeding in plants was established in the middle of the 20th century and is still practised today. In this process, random changes are artificially induced in the genetic material, thus broadening the genetic basis for breeding. In most cases, the plant seeds are exposed to ionising radiation (X-rays or neutron beams), which triggers mutations. The resulting mutants are examined for interesting genes or traits that can then be crossed into existing varieties. Worldwide, more than 3,000 new varieties of crops have already been bred with the help of radiation. The products include the majority of durum wheat varieties used to make pasta, many other cereals, varieties of fruit, vegetables, and legumes.
Instead of ionising radiation, newer methods use chemicals such as ethyl methanesulfonate, which can also modify genes. They can be combined with systematic DNA analyses to determine more quickly whether the desired gene change has been achieved. The researchers need to know beforehand, however, which changes are to be detected. The combination of chemical mutagenesis and subsequent genome screening is called TILLING, which stands for Targeting Induced Local Lesions in Genomes.
The era of conventional genetic engineering
Experiments on tobacco plants laid an important foundation stone for green genetic engineering in the 1980s. Researchers in Cologne/Germany and Ghent/Belgium succeeded for the first time in transferring a foreign gene into the genome of a plant. They used the soil bacterium Agrobacterium tumefaciens, which still plays an essential role in green genetic engineering today. This type of bacteria has the natural capability to infect plants and change their genome. Plasmids are used to do this. These circular DNA elements, which bacteria have in the chromosome in addition to their genome, can penetrate other cells across species boundaries. In genetic engineering, they can be used as a ferry, so to speak, to transfer genetic material. For this purpose, the plasmids are equipped with the desired gene in the laboratory.
This method makes it possible to transfer individual genes without the need for fertilisation. Traits can thus be transferred even between two plant species that cannot be crossed. The inserted genes cause, for example, the manifestation of a new trait or strengthen or weaken an already existing one.
These techniques have been used commercially since the mid-1990s, and one by one, transgenic crops appeared on the market. Among the first were soybeans tolerant to a particular herbicide, maize and cotton capable of repelling pests. Plants whose genome has one or more genes from a completely different organism inserted are called transgenic.
The insertion of additional genetic material with the help of bacterial plasmids is now considered conventional genetic engineering, as is the transfer of genetic material by gene gun. Here, tiny particles of gold or tungsten are loaded with the relevant genetic material. These constructs are introduced into the plant cells at high pressure. Afterwards, the DNA detaches from the particles and can insert itself into the genome of the cell nucleus. To monitor success, it is common practice to transfer marker genes as well, usually antibiotic resistance genes. Plant cells with this additional property grow on antibiotic-containing culture medium and can therefore be distinguished in the laboratory from plants where the gene transfer has failed.
The application of conventional genetic engineering
In the European Union (EU), green genetic engineering products have not yet been approved for cultivation, except for the corn variety MON810, which is resistant to the corn borer. In 2020, MON810 was only cultivated in Spain and Portugal on about 100,000 hectares. The global situation is different though: in 2019, genetically modified plants grew on a total of 190 million hectares, which is a good 13 per cent of the world’s arable land. Used are primarily varieties of soybeans, maize, cotton and rapeseed, which were developed using conventional genetic engineering methods. Among the countries where these products play a particularly significant role in agricultural production are the USA, Brazil, Argentina, Canada and India.
In India, genetically modified cotton plants account for 95 per cent of cotton production, and in Brazil, 95 per cent of soybean cultivation is done with genetically modified plants. The situation is similar in the USA, where more than 90 per cent of agricultural acreage for soybeans, maize and cotton is cultivated with genetically modified plants. So far, two traits are particularly significant for commercial crop cultivation: herbicide tolerance and resistance to plant feeding insects.
Herbicide tolerance makes plants insensitive to a specific weed control agent (herbicide). As a result, this agent can be used in the field to control weeds without endangering the cultivated product. A well-known example is soybeans, which are insensitive to the broad-spectrum herbicide glyphosate. This is achieved by inserting a gene from the soil bacterium Agrobacterium tumefaciens, which causes the plants to produce a different form of a specific enzyme (EPSP synthase). In its original form, this enzyme is the point of attack for glyphosate and therefore inhibited by the herbicide. Because EPSP synthase is essential for the formation of protein building blocks, plants stop growing after treatment with the agent and die after a few days. Glyphosate-resistant soybeans possess the bacterial form of EPSP synthase and are thus insensitive to the herbicide.
However, this concept also has drawbacks. Many weeds have become tolerant or even resistant to glyphosate, which makes it necessary to increase the dosage in some cases to still achieve high crop yields. Furthermore, the use of glyphosate is viewed critically concerning its consequences for biodiversity. In Germany, for example, the Federal Agency for Nature Conservation warned in a position paper published in 2018 of significant negative impacts on biodiversity, especially in the agricultural landscape.
In addition to herbicide tolerance, conventional green genetic engineering often aims to protect plants from plant feeding insects by modifying their genetic material. This is done, for example, by transferring the gene for the production of the so-called Bt protein. It originates from the soil bacterium Bacillus thuringiensis and occurs in different variants. It can protect maize from pests such as the corn borer moth’s caterpillars and the larvae of the corn rootworm, a beetle species. It also helps against insects that feed on cotton plants. A non-toxic form of the Bt protein is produced in the plant and subsequently converted to a toxic form within the intestines of the respective pests. It then binds to their intestinal walls, and they perish. Farmers can manage with significantly fewer insecticides as a result.
Genome editing: Precise interventions with genetic scissors
Genome editing tools, also known colloquially as genetic scissors, are various molecular systems with which individual building blocks of the genome of any living organism can be specifically and precisely rewritten – i.e. in a sense edited. This opens up many new possibilities not only for medicine but also for plant breeding. Here, they additionally allow time savings. Experts estimate that genome editing can save between six and 50 years compared with conventional breeding methods and depending on the plant species.
The term genome editing encompasses a range of methods that have been developed since the mid-1990s and include, in addition to the well-known genetic scissors CRISPR/Cas, the molecular systems TALEN, zinc-finger nucleases and meganucleases. These methods make it possible to target and sever almost any location in the genome sequence with pinpoint accuracy. Cutting the DNA strand can have the effect of inactivating or modifying a gene. It is also possible to insert a new gene at a specific location to change or supplement a trait. Meanwhile, researchers can even use base editors to specifically modify individual DNA building blocks without first cutting the DNA strand.
The programming of TALEN and zinc-finger nucleases is time-consuming and cost-intensive. The CRISPR/Cas method is comparatively efficient, thus saving time and costs. This is also the reason for its preferred use in many research and development laboratories today.
“In short, genome editing can be used to insert almost any mutation at any desired location in the genome, and it can be done much quicker, more efficient and precise than with conventional breeding methods. Mutations are, in a sense, the engine of breeding. They can lead to new desirable characteristics in crops, for example, higher yields or better taste. And such desirable mutations can either be isolated in a breeding programme that lasts for decades or they can now be specifically created within a few months time using the new methods. So breeding will become much faster and more precise with these new methods.”
Photo: MPI-MP
CRISPR/Cas: An immune system of bacteria
The abbreviation CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. This refers to short, repetitive DNA segments. They were first discovered in 1987 in the bacterium Escherichia coli. Twenty years later, it became evident that they also occur in numerous other bacteria and are part of an adaptive immune system that these organisms use to ward off viruses.
Viruses that can infect bacteria are called phages. They penetrate the bacterial cell and inject their genetic material. As a result, the bacteria produce new phages and usually perish. Some bacteria have a defence mechanism that protects them from phages. The CRISPR/Cas system serves them as an immune memory and defence at the same time. They integrate a short section of phage DNA into the CRISPR sequences of their genetic material. This creates a collection of all the pathogens that the cell has been confronted with for future recognition. It is also passed on to the bacterial offspring.
About half of all bacteria known today and almost all species formerly called primordial bacteria possess a CRISPR/Cas defence system. The best known, namely CRISPR/Cas9, is one of the most straightforward systems of its kind. The CRISPR sequences in the genetic material represent the blueprint for short molecules of ribonucleic acid (RNA), which then serve as pilot molecules and guide the other part of the genetic scissors, the Cas protein, to a specific location in the genome. By changing part of this sequence, the principle can be modified and used technically as genetic scissors that target specific points of the genome.
The system used in the lab basically consists of two parts: the CRISPR-derived guide RNA and the cutting tool Cas. The abbreviation Cas stands for CRISPR-associated proteins, which are enzymes. The frequently mentioned Cas9 is one example from this enzyme family. It was a major factor in the works on CRISPR in bacteria of the genus Streptococcus. There are also other Cas proteins, such as Cas12 and Cas13, found in different bacteria in nature.
CRISPR/Cas as a tool
2012 is considered a decisive year for the breakthrough of CRISPR/Cas technology. That year, the research groups of Emmanuelle Charpentier (now Max Planck Research Unit for the Science of Pathogens in Berlin/Germany) and Jennifer Doudna (University of California at Berkeley/USA) published their findings on how the natural mechanism by which bacteria recognise and repel invading viruses can be used for targeted interventions in the genetic material. In 2020, the two scientists were awarded the Nobel Prize in Chemistry for developing this genome editing method.
After Charpentier and Doudna published their experiments with the genetic scissors on bacteria, the far-reaching significance of their findings quickly became evident. Other research teams showed that the CRISPR/Cas9 genetic scissors can also be used on the genome of other organisms, including animals, humans and plants. What is interesting about this molecular tool is not only its ease of use, but also the fact that it utilises the cells’ own repair mechanisms. These are set in motion by cutting the DNA strands and can be used to inactivate a gene, change individual building blocks or even insert larger DNA segments.
In general, there are two paths that can be taken after the DNA strand has been cut. It depends on whether the genetic scissors are introduced together with a DNA sequence similar to the target site as a repair template, which is to be specifically incorporated into the genome of the targeted cells. So far, CRISPR/Cas9 has mainly been used in plant research to modify or deactivate individual genes selectively. This alone opens up a broad spectrum of possibilities for generating plants with new traits relatively quickly.
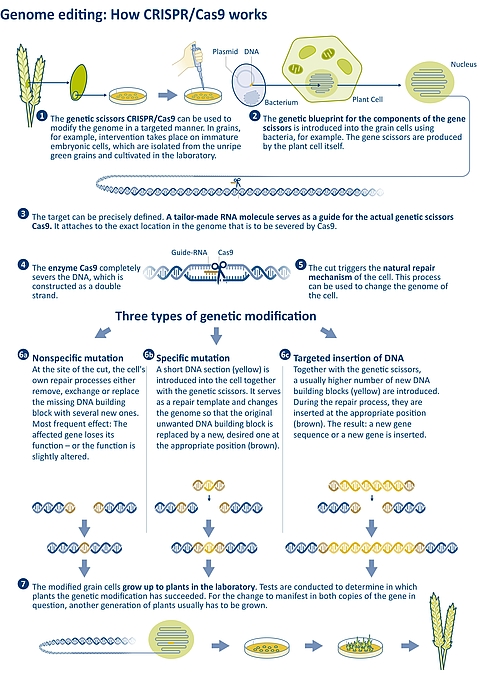
Figure 3: Operation principle of the CRISPR/Cas9 genetic scissors: This genome editing method can be used to precisely target and sever specific sites in the genome. The repair process triggered thereby can be used to create genetic changes (mutations) or to insert entire genes. | Source: JKI/IPK | Illustration: Emde Grafik
In the absence of a repair template, the joining of the cut ends is nonspecific. While the cell tries to simply reconnect the strand, small errors can occur in the process. For example, individual genomic building blocks may get lost, or new ones may be added. When using genetic scissors, these small errors are intentional because the gene in question thereby loses its function and is switched off. Another result may be a slightly altered gene function. What precisely happened in the genome after the targeted cut must subsequently be examined in the laboratory. This procedure (non-homologous recombination) is not completely specific, but nevertheless much more targeted than procedures of conventional genetic engineering or mutagenesis breeding, where it is often not possible to determine in advance at which point in the genome a change is to take place (see Figure 3, 6.1).
Homologous recombination is more specific. In this process, genetic scissors are used to transfer an additional DNA fragment to be incorporated into the genome. The DNA fragment has a sequence at both ends that match the gene building block sequence at the ends of the broken strand. This leads to the cell being able to repair the severed DNA strand using this template. In homologous recombination, either one or just a few gene building blocks can be exchanged, or an entire gene is inserted. (see Figure 3, 6.2 and 6.3).
“What I believe is often not taken into account, but extremely important, is the fact that genome editing helps us understand gene function in an unprecedented way – that is, in cultivated species and not just in model species. This is a great knowledge gain that can be directly applied to breeding. For this we also need, for instance, field trials or a similar system where research in this area can actually be verified.”
How other genetic scissors work
The existence of enzymes like Cas, which can sever the DNA strands, has been known for some time. The first potential genetic scissors of this kind were discovered more than 40 years ago. Only recently, however, had such enzymes been made programmable – i.e. directing them precisely to the place where they are supposed to cut the genome.
The CRISPR/Cas system has a decisive advantage over other genetic scissors: The guide RNA is easy to construct. It only consists of about 20 genetic building blocks that can be easily produced in the laboratory. For the genetic scissors TALEN and zinc-finger nucleases, on the other hand, a complete protein has to be produced that determines the location where the genome is to be cut. This takes longer, is more expensive and often less efficient.
- The TALEN genetic scissors are based on a protein that occurs in Xanthomonas bacteria and is modified to suit these genetic scissors. TALEN stands for Transcription Activator-Like Effector Nuclease. The protein leading to the desired target sequence in the genome works very precisely and can also be programmed for longer target sequences.
- Zinc-finger nucleases can also be used to edit the genetic material. They consist of the zinc-finger protein, which recognises a specific sequence in the genome, and an enzyme that cuts the DNA strands: a nuclease. The zinc-finger protein must be artificially produced in a relatively complex process.
- Meganucleases are genetic scissors based on naturally occurring enzymes that are modified accordingly. They are more accurate than zinc-finger nucleases because they recognise longer sequences. However, their adaptation to new target sequences is so complicated that meganucleases are hardly used in research and application.
- The genomic method of oligonucleotide-directed mutagenesis (ODM) is used in basic research and plant breeding. In this process, short DNA segments, oligonucleotides, are constructed and introduced into the genome. They bind to the intended site and trigger a so-called mismatch there. This irregularity activates the cell’s own repair mechanisms, which use the inserted molecule as a repair template and copy the inserted information into the plant’s genome without inserting the foreign DNA. This allows for individual building blocks of genetic material to be exchanged and short sequences to be inserted or deleted. The ODM principle was used in 1999 to modify the first cultivated plants genetically.
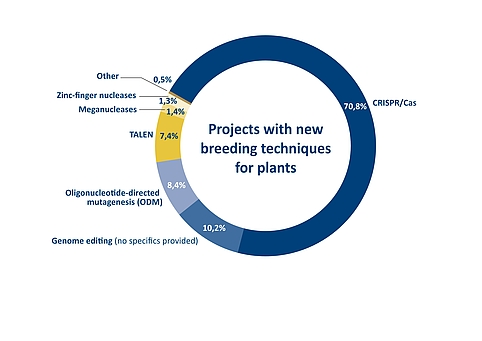
Figure 4: Proportion of different genome editing methods in projects for developing plants with modified or new traits. The overview of a total of 426 projects worldwide was compiled for a report by the Joint Research Centre of the European Commission. Source: Parisi, C., Rodrigeuz-Cerezo, E., Current and future market applications of new genomic techniques, Luxembourg, 2021 | Illustration: Emde Grafik
The extent to which CRISPR/Cas technology meanwhile dominates plant breeding is reflected in the EU Commission’s Joint Research Centre/JRC report on current and future market applications of new genomic techniques, published at the end of April 2021. It provides an overview of 426 genome edited products in total, ranging from already approved products to projects in the early stages of research and development. According to the technology used in each case, the breakdown shows that more than 70 per cent of the projects are based on the CRISPR/Cas method. TALEN and ODM account for around seven and eight per cent, respectively.